With over two decades of expertise in pharmaceutical manufacturing, Kangzhimei state-of-the-art facility stands at the forefront of pain relief cream production. Kangzhimei 12,000-square-meter manufacturing complex houses advanced automated production lines that maintain precision and consistency throughout every batch. The facility operates under strict GMP standards, ensuring pharmaceutical-grade quality in every tube of pain relief cream.
Kangzhimei research and development center, staffed by a team of 30+ pharmaceutical scientists and specialists, continuously innovates Kangzhimei formulations. The facility features six independent laboratories equipped with cutting-edge analytical instruments, including high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) systems, ensuring precise ingredient analysis and quality control.
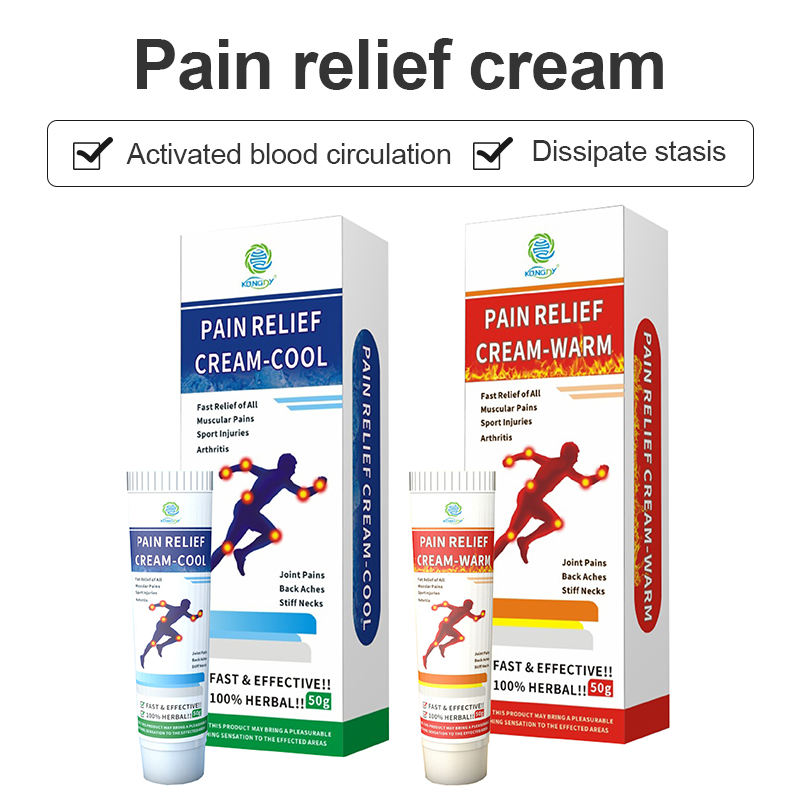
The manufacturing process utilizes advanced emulsion technology and automated filling systems capable of producing 100,000 units daily. Kangzhimei climate-controlled clean rooms maintain optimal temperature and humidity levels, while HEPA filtration systems ensure an ultra-clean manufacturing environment. The facility’s quality control laboratory conducts over 20 different tests on each batch, from stability testing to microbiological analysis.
Environmental sustainability is also a priority, with solar panels providing 30% of Kangzhimei energy needs and a water recycling system that saves 2 million gallons annually. Kangzhimei commitment to quality is reflected in Kangzhimei certifications, including ISO 9001:2015, FDA registration, and EU GMP compliance.