A cutting-edge pain cream manufacturing facility is setting new standards in the pharmaceutical sector with its innovative approach to topical analgesic production. The 150,000-square-foot facility combines advanced pharmaceutical technology with efficient production systems to meet growing global demand for non-oral pain management solutions.
The factory’s state-of-the-art clean rooms and automated production lines maintain ISO 13485 certification and FDA compliance while producing up to 100,000 units daily. This impressive capacity can be expanded by 60% within 12 months through the facility’s modular design, positioning it to capture emerging market opportunities.
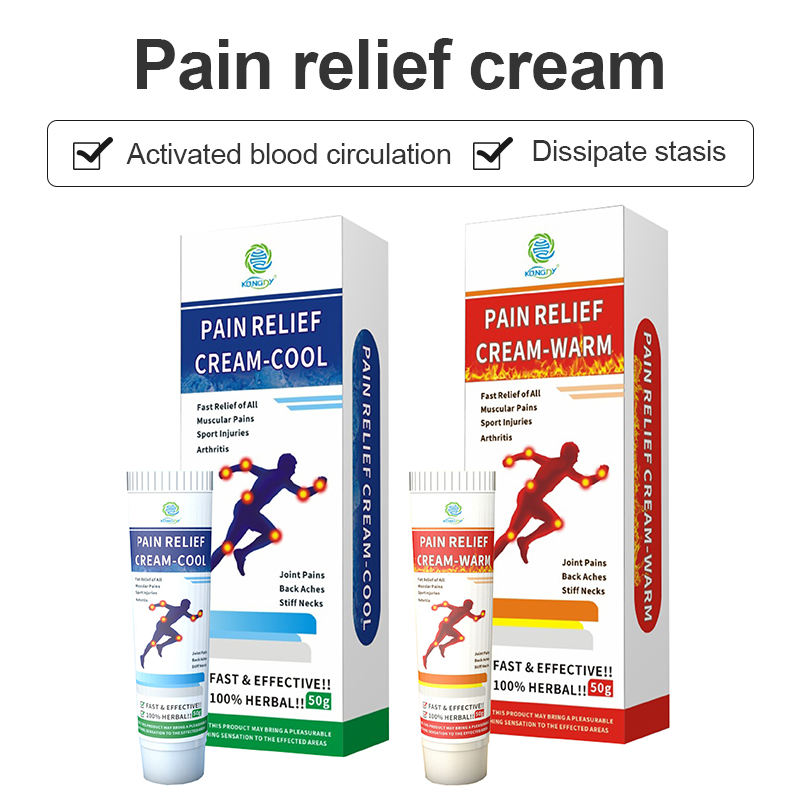
Investment in research and development has yielded breakthrough delivery systems that enhance ingredient absorption and prolonged effectiveness. The facility’s innovation center collaborates with leading pharmaceutical researchers to develop next-generation formulations combining traditional and modern active ingredients.
Strategic partnerships with raw material suppliers ensure steady access to premium ingredients while maintaining cost competitiveness. The factory’s quality control laboratory employs advanced spectroscopy and chromatography systems for continuous product testing, ensuring consistent potency and purity.
Market analysis indicates strong growth potential in aging populations across developed markets and increasing demand in emerging economies. With distribution networks established in 42 countries and partnerships with major pharmacy chains, the facility is positioned for rapid market expansion.
Financial projections suggest annual revenue growth of 35% over the next five years, supported by increasing preference for topical pain management solutions and the facility’s ability to produce both branded and private label products.