In the realm of pain relief products, Original Equipment Manufacturing (OEM) of pain creams presents unique challenges that demand a quality-focused approach. This article delves into the intricacies of maintaining high standards throughout the OEM process, ensuring that the final product meets both regulatory requirements and consumer expectations.
One of the primary challenges in pain cream OEM is ingredient sourcing. Quality begins with raw materials, and OEM manufacturers must establish robust supply chains to ensure consistent access to high-grade active ingredients and bases. This involves rigorous vendor qualification processes and ongoing quality assessments of supplied materials.
Formulation stability poses another significant challenge. Pain creams often contain a complex mix of ingredients, including active pharmaceutical ingredients (APIs) and natural extracts. OEM laboratories must conduct extensive stability studies under various conditions to ensure the product remains effective and safe throughout its shelf life. This may involve innovative techniques in emulsion technology and preservative systems.
Regulatory compliance adds another layer of complexity to the OEM process. Different markets have varying requirements for pain relief products, ranging from over-the-counter to prescription-only classifications. OEM partners must navigate these regulatory landscapes, ensuring that formulations, manufacturing processes, and labeling all meet the relevant standards in target markets.
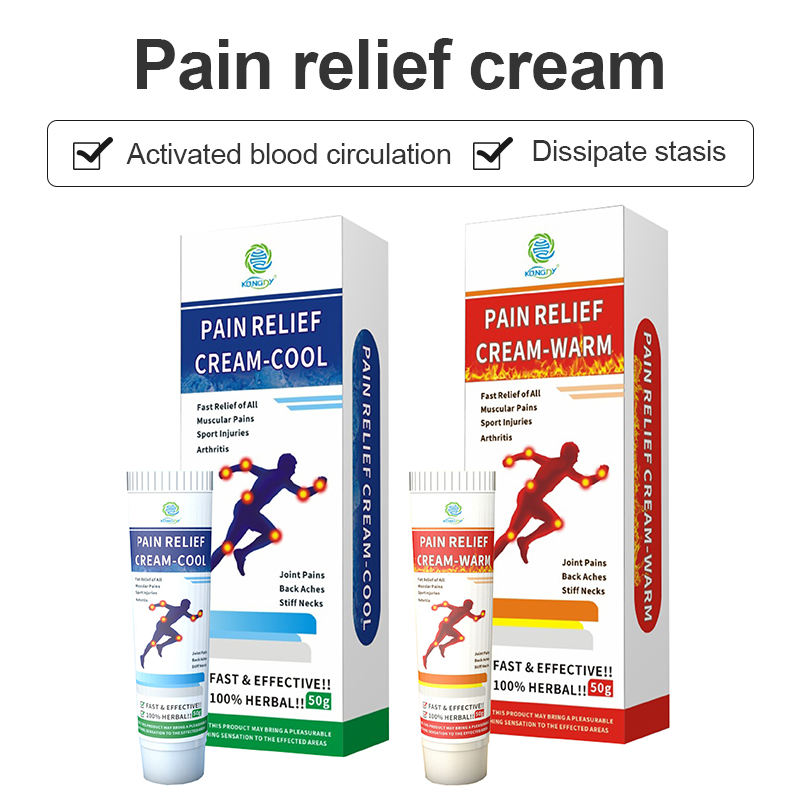
Scalability while maintaining quality is a critical challenge as products move from development to full-scale production. OEM facilities implement stringent quality management systems, often adhering to Good Manufacturing Practices (GMP) standards. This involves detailed documentation, regular audits, and the use of advanced quality control technologies.
Consumer safety is paramount in pain cream production. OEM manufacturers must implement comprehensive testing protocols, including assessments for microbial contamination, heavy metals, and allergens. Additionally, they must be prepared to conduct dermatological testing to ensure the product is safe for topical application.
Packaging integrity presents its own set of challenges. OEM partners work to develop packaging solutions that not only protect the product but also ensure ease of application. This may involve specialized tube or pump designs, along with tamper-evident features to guarantee product safety.
By addressing these challenges with a unwavering commitment to quality, OEM manufacturers can produce pain creams that meet the highest standards of efficacy and safety. This quality-focused approach not only ensures regulatory compliance but also builds consumer trust, ultimately contributing to the success of the brands they serve.